
Kelly Grant
Dec. 9, 2020
Health Canada has granted interim authorization to a COVID-19 vaccine made by Pfizer Inc., and its German partner, BioNTech. On Tuesday, scientists from the U.S. Food and Drug Administration released a briefing document analyzing the clinical trial evidence for the vaccine. The FDA document, which provides the first in-depth look at the data for the Pfizer-BioNTech shot, was released ahead of a Thursday meeting of outside experts who will evaluate the shot for the U.S. market. Here’s what it tells us about the vaccine that Canadians could start taking next week.

HOW EFFICACIOUS IS THE VACCINE?
Very, according to the results of Pfizer-BioNTech’s phase 3 trial. The trial involved about 44,000 people, half of whom were given the vaccine, half the placebo. The trial was double-blinded, meaning neither the people getting the shots nor the people giving them knew what was in the syringe.
Pfizer’s vaccine is administered as two doses 21 days apart. The trial’s primary goal was to see if the shot prevented mild to severe COVID-19 in people who hadn’t already been infected with SARS-CoV-2, the virus that causes COVID-19.
The trial’s clock began ticking seven days after the participants — who were asked to go about their daily lives as normal — received their second dose. On Nov. 18, Pfizer and BioNTech announced that more than enough of the trial participants had contracted COVID-19 to reach a conclusion. Of 170 confirmed cases of COVID-19, 162 were in the placebo group. Eight were in the vaccine group. That works out to 95 per cent efficacy against disease.
One of the eight cases in the vaccine group was defined as “severe,” but the FDA analysis revealed the patient only met the criteria because his or her oxygen saturation slipped below normal for a short period during a COVID-19 illness appointment. The patient wasn’t admitted to hospital and didn’t seek further medical care.
WHO WAS THE VACCINE TESTED ON?
The new FDA documents provide more details about the kinds of people who took part in the trial, which was conducted in the U.S., Germany, Turkey, South Africa, Brazil, and Argentina. Ten per cent were African American, 26 per cent Hispanic and 4.4 per cent were Asian. The vaccine was tested on people with some of the underlying conditions that have been associated with a greater risk of getting seriously sick with COVID-19: Thirty-five per cent of participants were obese, 8.4 per cent had diabetes and 7.8 per cent had pulmonary disease.
One big concern has been how well Pfizer’s vaccine protects the elderly, who are most at risk of bad outcomes from COVID-19 and, based on evidence from other vaccines, the least likely to mount a strong immune response to the shot. The trial enrolled just over 1,700 people aged 75 and older. There were no cases of COVID-19 detected in people over 75 who received the real shot, and five in that age cohort of the placebo group.
“Is that enough to give me full confidence in that specific cohort to say that it’s effective there? Not necessarily,” said Srinivas Murthy, a pediatric infectious diseases physician at BC Children’s Hospital and expert in clinical trial design. “But with the overall effect estimate being so strong, the balance of evidence suggests a benefit.”
IS THE VACCINE SAFE?
When it comes to “serious adverse events” — significant health problems experienced during the trial — the frequency was low and, as the FDA put it, “without meaningful imbalance between study arms.”
Alyson Kelvin, a virologist and assistant professor at Dalhousie University currently working on COVID-19 vaccines at the University of Saskatchewan’s VIDO-InterVac, said that that means, “there weren’t any major differences in the reported serious adverse events in the placebo and vaccinated groups. That’s compelling.”
Mild to moderate reactions are another story. Injection site reactions occurred in 84.1 per cent of recipients, fatigue in 62.9 per cent, headache in 55.1 per cent, chills in 31.9 per cent, joint pain in 23.6 per cent and fever in 14.2 per cent. That makes sense, considering the vaccine’s purpose is to nudge the body’s immune system into defensive mode, Dr. Murthy said.
“This vaccine does cause a reaction. I think that should be the message that we tell patients when we’re giving this vaccine,” Dr. Murthy said. “We don’t want people to walk away from their vaccine saying, ‘Ow, that hurt, I didn’t like it.’ We should definitely prepare them that it’s going to cause some discomfort.”
The FDA scientists flagged that 64 recipients of the vaccine reported temporarily swollen lymph nodes, far more than six in the group that took a dummy shot. They also noted that four cases of Bell’s palsy, a temporary drooping of muscles in one side of the face, occurred in the vaccine group. None occurred in the placebo group. In the four cases, Bell’s palsy symptoms cropped up at three, nine, 37 and 48 days after vaccination. The first case — the one that showed up three days after a shot — was reported as resolved three days after it began and the others were “continuing or resolving” as of the middle of November.
Zain Chagla, an infectious disease physician and associate professor at McMaster University, said it could be a coincidence that the four cases showed up in the vaccine group. “When you’re talking about an event [Bell’s palsy] that happens in 1 in 10,000 to 1 in 20,000 individuals, it just might be chance that you had a couple in one group and none in the other,” he said. “It’s certainly something that needs to be monitored in post-marketing follow-up.”
The FDA analysts agreed, writing that the rate of Bell’s palsy in the trial is about what you’d expect in the general population. Still, they recommended surveillance of Bell’s palsy cases as the vaccine rolls out.
COULD THE VACCINE PROVIDE SOME PROTECTION AFTER JUST ONE DOSE?
It looks like it, but experts still recommend getting the booster dose three weeks after the first shot. In the first week or so after the initial dose, the fates of the vaccine and placebo groups diverged in a way that suggests the first shot alone could be a little over 50 per cent efficacious, though not right away.
In the interval between the first and second doses, 82 people in the placebo group developed COVID-19, while only 39 people in the vaccine group did. Still, protection was far stronger seven days or more after the second dose.
On the whole, Dr. Murthy said he was impressed with Pfizer and BioNTech’s study, praise he doesn’t toss around lightly. “I’m always the first to be skeptical of studies and skeptical of results and trying to find things to nitpick before implementation,” he said, “but this data is pretty robust.” The FDA scientists agreed, indicating the evidence supports granting Pfizer-BioNTech’s shot emergency use authorization, something that is likely to happen by the end of this week.
ARE THERE UNKNOWNS AS WE PREPARE TO ROLL OUT THIS VACCINE?
Yes. We know that Pfizer’s shot protects against COVID-19 disease, but we don’t know if it prevents asymptomatic infections and silent spreading. We don’t know how long the immunity conferred by the vaccine lasts. That’s something researchers and regulators will be watching closely; trial participants will be followed for another two years. We don’t know if the shot is safe for pregnant women. They were excluded from the trial, as were children until the age of 12. Some younger teens were added to the trial as it progressed, but not enough to draw conclusions yet.
HOW DOES THE VACCINE ACT ON THE BODY?
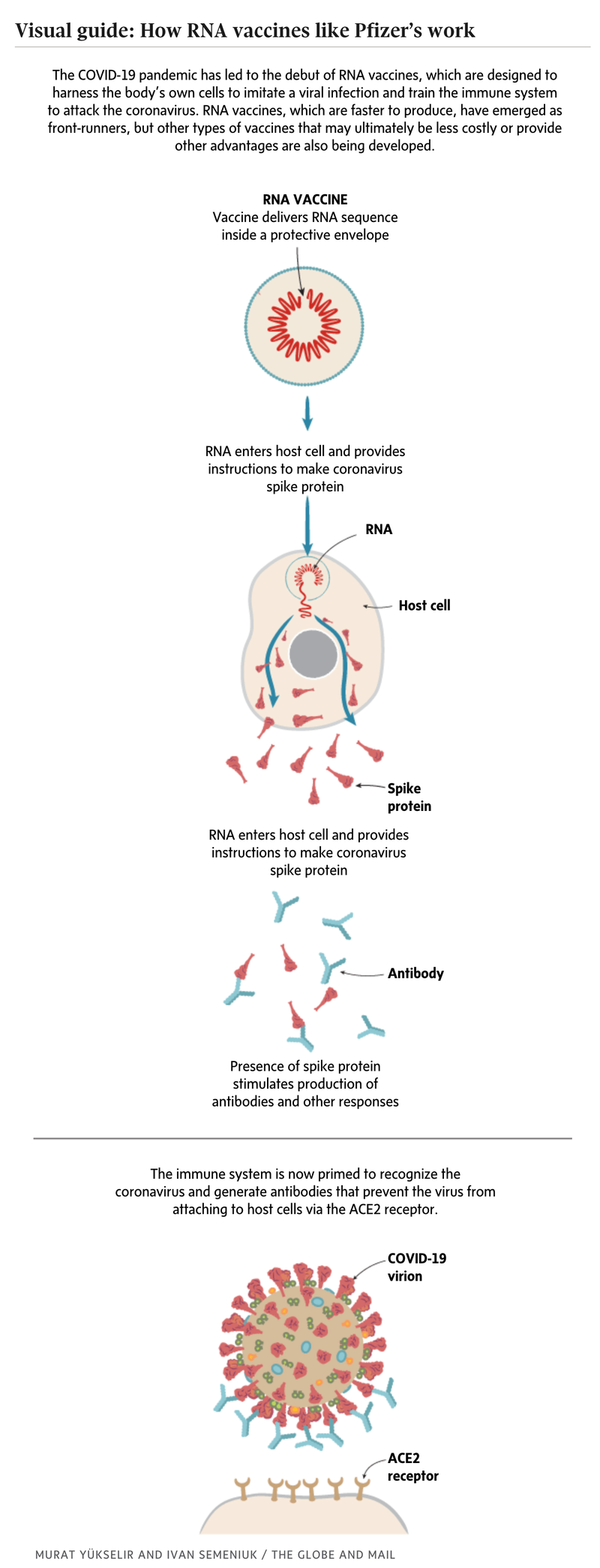
The vaccine is one of the first RNA vaccines. Instead of delivering an inactivated virus or viral proteins to teach the body’s immune system what to watch for, it enlists the body’s cellular machinery to make those proteins on its own.
But while the vaccine consists of a portion of the genetic code of the virus, it cannot reconstitute the virus, only the part of the virus called the spike protein, which the immune system recognizes as the calling card of an infection.
The vaccine also does not involve or affect the DNA of those who receive it. Instead it bypasses the DNA and simply instructs cell to make the necessary protein, based on the genetic instructions it delivers — just as the coronavirus does.
Once the protein is made, it is spotted by immune cells and the body’s infection-fighting system kicks in. This is why the vaccine may temporarily cause those who receive it to feel like they are getting sick. Afterwards, the immune system returns to normal but it is now trained to recognize and fight the coronavirus whenever it appears for as long as immunity lasts.
This Globe and Mail article was legally licensed by AdvisorStream.
© Copyright 2024 The Globe and Mail Inc. All rights reserved.


