By Dominique Mosbergen
Aug. 28, 2023
Scientists obsessed with aging are sketching a road map of how our bodies change as we grow old in the hopes that it will lead to treatments that could help us live longer, healthier lives.
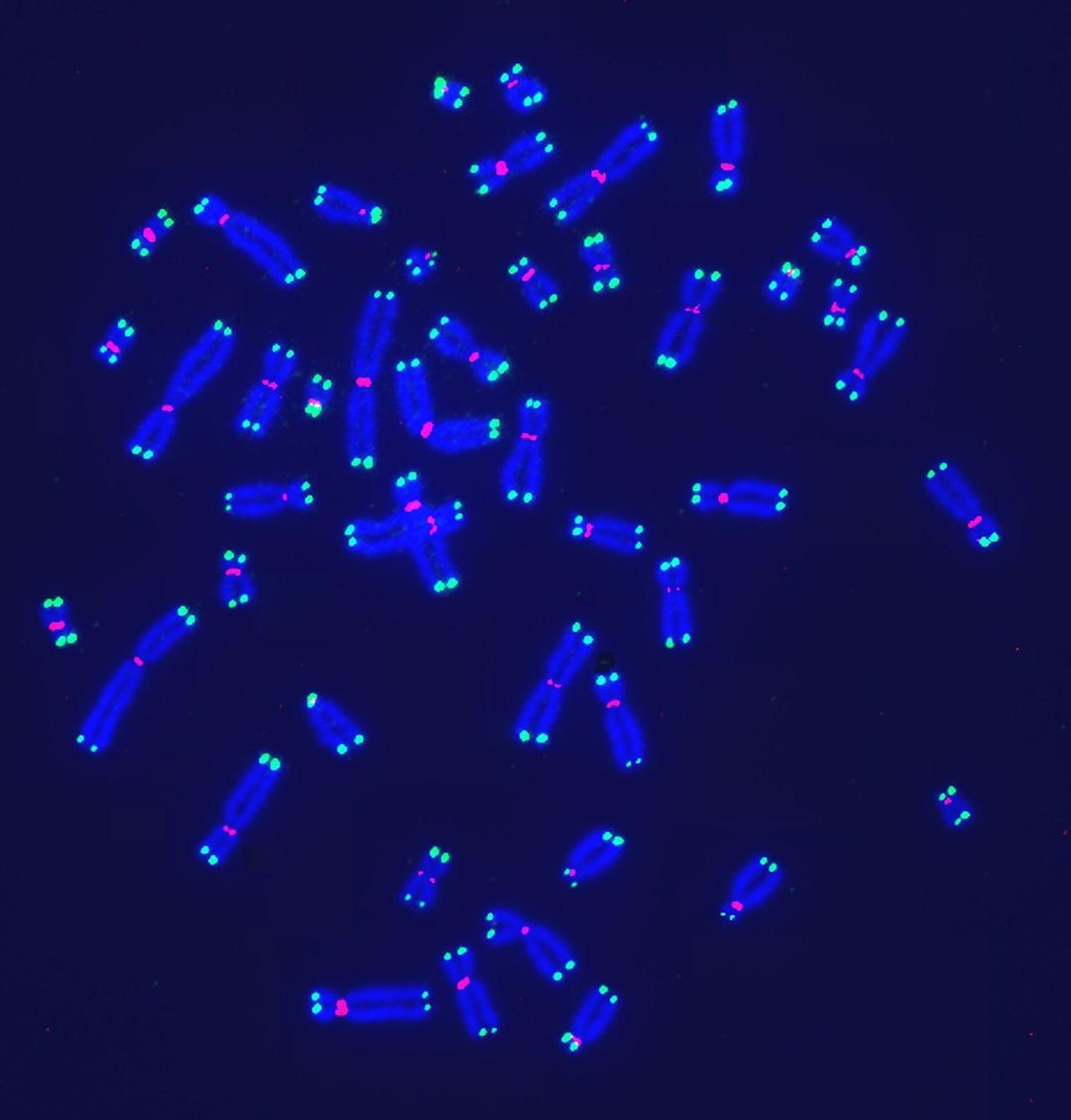
A visualization of telomeres—DNA segments at the ends of chromosomes—using digital fluorescence microscopy. PHOTO: SHAY LAB/UT SOUTHWESTERN MEDICAL CENTER
They call this road map the “hallmarks of aging”—a set of biological features and mechanisms linked to our inexorable march toward death. Over the past decade, the hallmarks have helped guide the development of drugs that clear away cells that have stopped dividing and gene therapies that appear to restore cells to a more youthful state.
Scientists in Europe codified nine hallmarks in a 2013 paper in the journal Cell that is widely cited in the aging field. They include: shortening of telomeres (DNA segments at the ends of chromosomes); cell senescence, when cells stop dividing; and breakdowns in how cells regulate nutrients.
The hallmarks appear to manifest with age and accelerate aging when enhanced. They are interconnected in ways researchers are trying to understand. Some believe this could unlock insights into why we age.
Scientists are getting closer to solving mysteries that have long vexed thinkers. Gilgamesh, the titular character of an epic poem etched some 4,000 years ago on clay tablets, was obsessed with overcoming mortality. Chinese Emperor Qin Shi-Huang, who died in 210 B.C., drank mercury hoping to cheat death.
“Aging has excited the imagination throughout the history of humankind,” said Carlos Lopez-Otin, a biochemist at the University of Oviedo in Spain who co-wrote the hallmarks paper, “But it’s only recently that it has been subjected to profound scientific scrutiny.”
One hallmark attracting attention is changes in the epigenome, which consists of chemical compounds and proteins that can attach to DNA and regulate whether genes are turned on or off. Some researchers think an accumulation of errors in the epigenome drives aging and that removing the errors by “reprogramming” cells could lengthen life.
Shinya Yamanaka, a Japanese stem-cell researcher, shared a Nobel Prize in 2012 for discovering proteins that reprogram a cell’s epigenome to its embryonic state.
Scientists have used the proteins to extend the lifespan of mice and reverse blindness in mice and monkeys. Biotechnology companies such as Altos Labs, which Yamanaka advises, Retro Biosciences and Calico Life Sciences, part of Google parent Alphabet, are probing whether cellular reprogramming could extend lifespans or improve health.
Dorian Therapeutics and Senolytic Therapeutics are developing drugs that eradicate or prevent the formation of senescent cells, another hallmark, to see if that slows aging and mitigates age-related diseases.
Other scientists are experimenting with drugs targeting a hallmark called nutrient-sensing pathways: sensors that cells use to recognize fuel sources such as sugars and proteins. The sensors become less effective with age and their deterioration has been linked to myriad health problems including metabolic disorders and cancer.
Nutrient-sensing pathways are affected by restricted diets, which research shows can bolster longevity. Diets that significantly cut calories increase lifespans and improve health, studies have shown. One theory is that calorie restriction stresses cells and increases their resilience.
Drugs including rapamycin that appear to mimic the effects of calorie restriction have increased life expectancy in mice. Rapamycin, which blocks a type of nutrient sensor, is typically taken by organ-transplant recipients to suppress the immune response.

The Dog Aging Project is testing whether rapamycin can extend lifespan in pet dogs. PHOTO: DOG AGING PROJECT
The Dog Aging Project, headquartered at the University of Washington, is testing whether rapamycin can extend lifespan in pet dogs. AgelessRx and researchers at Columbia University are among the groups helping to develop clinical trials to test rapamycin’s antiaging potential in people.
A popular theory that emerged in the past century held that telomeres, another hallmark, could offer a silver-bullet solution to aging. Researchers who discovered the molecular nature of telomeres and telomerase, an enzyme that can maintain or extend their lengths, won a Nobel Prize in 2009.
Telomeres shorten as people age, and shorter telomeres appear to be associated with disease, studies show. But it isn’t clear that extending telomeres would lengthen life. Activating telomerase can allow cancer cells to replicate unchecked. People who have unusually long telomeres have an increased risk of developing tumors and a blood disorder, according to a study published in May in the New England Journal of Medicine.
Still, some researchers and companies including Telomere Therapeutics and Geron are manipulating telomeres in efforts to treat cancers and other age-related disorders.
“It’s like Dr. Jekyll and Mr. Hyde: We want to stop telomerase in cancer cells…but elongating telomeres safely could be useful for a variety of age-related conditions,” said Jerry Shay, a molecular biologist at the University of Texas Southwestern Medical Center in Dallas and co-founder of Telos Biotech, which is lengthening telomeres to try to improve immune function in cancer patients.
Researchers of aging said the hallmarks have helped shape a shared vision for the field. But while they describe some of what happens during aging, they don’t explain why these changes occur, said David Gems, a geneticist at University College London.
They fall short of what philosopher Thomas Kuhn defined in the 1960s as a paradigm: shared values and ideas that explain a scientific phenomenon. “You can’t have a field without a paradigm,” Gems said.
The group behind the original hallmarks of aging suggested three more earlier this year based on subsequent research: chronic inflammation; imbalance in the microbiome, the community of microorganisms that live inside people; and defects in autophagy, a cell’s ability to recycle damaged parts of itself.
More hallmarks will likely emerge and others will be discarded or combined as research advances, said Danish geneticist Lene Juel Rasmussen, who suggested a few others with colleagues last year.
“The hallmarks are dynamic,” Rasmussen said.
Nine Hallmarks of Aging
Here are some of the key biological changes identified by researchers that appear to happen with age
Genomic instability: As DNA damage builds up over time, mutations accumulate in the genome
Telomere attrition: Telomeres, protective structures at the ends of chromosomes, have been found to shorten
Epigenetic alterations: Processes that regulate whether genes are turned on or off can change over time
Loss of proteostasis: Machinery in cells that controls protein synthesis, maintenance and cleanup becomes impaired
Deregulated nutrient-sensing: Sensors that cells use to regulate fuel sources such as glucose become less effective
Mitochondrial dysfunction: Mitochondria, the power plants of cells, can become damaged and dysfunctional
Cellular senescence: Cells that stop dividing but don’t die accumulate as people age
Stem-cell exhaustion: Stem cells, which can develop into many cell types and serve as bodily repair systems, lose their regenerative power and other functions
Altered intercellular communication: Cells can lose the ability to properly communicate with each other
Write to Dominique Mosbergen at dominique.mosbergen@wsj.com
Dow Jones & Company, Inc.